While gels are generally composed of polymers (large molecules), supramolecular gels are formed by low-molecular-weight compounds connected through non-covalent bonds. In living organisms, the ability of non-covalent bonds to easily form and break is skillfully utilized in various biological activities. This property makes supramolecular gels promising next-generation functional materials for applications such as drug delivery systems that transport medicines to specific affected areas, artificial tissue materials that replace damaged ones, and environmental materials that absorb harmful substances.
A research team led by Lecturer Shinya Kimura and Professor Masamichi Yamanaka from Meiji Pharmaceutical University, Professor Takayuki Uchihashi from Nagoya University, Associate Professor Shinnosuke Kawai from Shizuoka University, and Professor Shiki Yagai from Chiba University, in collaboration with Teikyo University of Science, CONFLEX Corporation, and the Institute for Molecular Science, has become the first in the world to capture the detailed formation process of supramolecular gels at the nanoscale as a video, revealing the mechanism of its formation. Their findings were published in Nature Communications.
Credit: S. Kimura, K. Adachi, Y. Ishii, T. Komiyama, T. Saito, N. Nakayama, M. Yokoya, H. Takaya, S. Yagai, S. Kawai, T. Uchihashi and M. Yamanaka. CC BY
Previous research has suggested that supramolecular gels form through a process where: (1) low-molecular-weight gelators connect via non-covalent bonds to form thin fibers (fibrils), (2) these fibrils bundle together to create thicker fibers, and (3) these fibers form a network structure that entraps liquid to create a gel. However, the detailed mechanisms of how fibrils and fibers form and grow have remained unclear. Since the properties of supramolecular gels strongly depend on the characteristics of these fibers, understanding the mechanism would enable control over the physical properties and functions of supramolecular gels, greatly advancing the development of functional materials.
The research team successfully observed the growth process of fibers that constitute supramolecular gels as a "video" using high-speed atomic force microscopy. Contrary to previous assumptions, their observations revealed that thin fibers (fibrils) were not present; instead, thick supramolecular fibers grew directly from the beginning. Furthermore, they discovered that fiber growth does not proceed continuously but repeats a pattern of repeated elongation and pause phases.
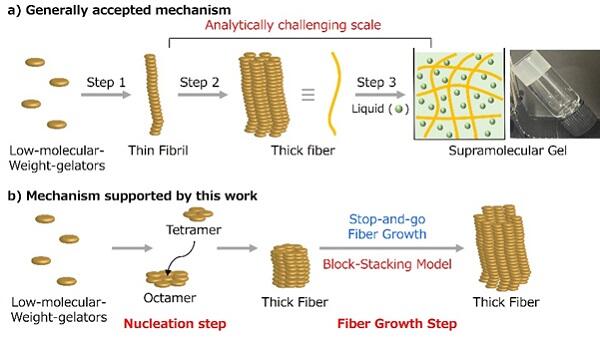
Credit: S. Kimura, K. Adachi, Y. Ishii, T. Komiyama, T. Saito, N. Nakayama, M. Yokoya, H. Takaya, S. Yagai, S. Kawai, T. Uchihashi and M. Yamanaka. CC BY
To explain this curious movement pattern, the research team proposed a new theory called the "block-stacking model." According to this theory, molecules align like building blocks and stack up at the tip of the fiber, causing the fiber to elongate upward. When the fiber tip has an irregular surface, newly attaching molecules can form stabilizing non-covalent bonds with adjacent molecules, making them more likely to stick and promoting growth. Conversely, when the fiber tip becomes uniformly flat, new molecules have difficulty attaching, temporarily halting growth. The research team conducted computer simulations based on this mechanism and confirmed that they could reproduce the observed repeated elongation and pause pattern.
The team also investigated how fiber formation initially begins. Through detailed image analysis of the gelation process, they revealed that it occurs in two distinct steps: first, a nucleus forms from a small number of molecules, and then the fiber grows as other molecules bind to this nucleus. Through statistical analysis of the gelation duration, they were able to estimate how many molecules constitute this nucleus. Through this research, they elucidated the complete process of how fibers that constitute supramolecular gels form and grow.
This achievement is expected to dramatically advance supramolecular gel research, eventually enabling free control of the properties of supramolecular gels.
Journal Information
Publication: Nature Communications
Title: Molecular-level insights into the supramolecular gelation mechanism of urea derivative
DOI: 10.1038/s41467-025-59032-6
This article has been translated by JST with permission from The Science News Ltd. (https://yz-jsjc-gov-cn-1416.res.gxlib.org.cn:443/rwt/1416/https/PNSXTLLPMW5YGLUDN6YGV6A/). Unauthorized reproduction of the article and photographs is prohibited.




